This is a Clilstore unit. You can .
Some curiosities to start.
Water is made up of two elements, hydrogen and oxygen. Its chemical formula is H2O.
The longest river in the world is the Nile river, it reaches 6650 kilometers in length .
While most people know that water boils at 100 °C, this is at the normal conditions of sea level. The boiling point of water actually changes relative to the barometric pressure. For example, water boils at just 68 °C on the top of Mount Everest while water deep in the ocean near geothermal vents can remain in liquid form at temperatures much higher than 100 °C.
Water expands as it cools from 4 °C to 0 °C (above 4 °C it does the opposite). In freezing conditions, water has been known to burst water pipes as it freezes to ice. That's why icebergs flotes
Water can move up narrow tubes against the force of gravity in what is known as capillary action.
Most people around the world have access to clean drinking water but it is a major problem in poorer areas of the world. Water pollution and low quality water can lead to dangerous bacteria, disease and viruses.
Drinking water is needed for humans to avoid dehydration, the amount you need each day depends on the temperature, how much activity you are involved in and other factors.
An important use for water is in agricultural irrigation, this is when water is artificially added to soil in order to assist the growth of crops.
Electricity can be created from hydropower, a process that uses water to drive water turbines connected to generators. There are many hydroelectric power stations around the world.
Water's properties
- Capillary action:
Capillary action occurs because water is sticky, thanks to the forces of cohesion (water molecules like to stay close together) and adhesion (water molecules are attracted and stick to other substances). Adhesion of water to the walls of a vessel will cause an upward force on the liquid at the edges and result in a meniscus which turns upward. The surface tension acts to hold the surface intact. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. The height to which capillary action will take water in a uniform circular tube (picture to left) is limited by surface tension and, of course, gravity.

- Cohesion and adhesion:
Cohesion: Water is attracted to water
Adhesion: Water is attracted to other substances
It is easy to see that the drop seems to have a "skin" holding it into a sort of flattened sphere (although there is nothing flat about a water drop in outer space.). It turns out that this surface tension is the result of the tendency of water molecules to attract one another. The natural form of a water drop occurs in the "lowest energy state", the state where the atoms in the molecule are using the least amount of energy. For water, this state happens when a water molecule is surrounded on all sides by other water molecules, which creates a sphere or ball (perfectly round if it was in outer space). On Earth, the effect of gravity flattens this ideal sphere into the drop shape we see. Although you may have heard of a "skin" where water meets the air, this is not really an accurate description, as there is nothing other than water in the drop.
- Surface tension:
The cohesive forces between liquid molecules are responsible for the phenomenon known as surface tension. The molecules at the surface of a glass of water do not have other water molecules on all sides of them and consequently they cohere more strongly to those directly associated with them (in this case, next to and below them, but not above). It is not really true that a "skin" forms on the water surface; the stronger cohesion between the water molecules as opposed to the attraction of the water molecules to the air makes it more difficult to move an object through the surface than to move it when it is completely submersed.

- Density:
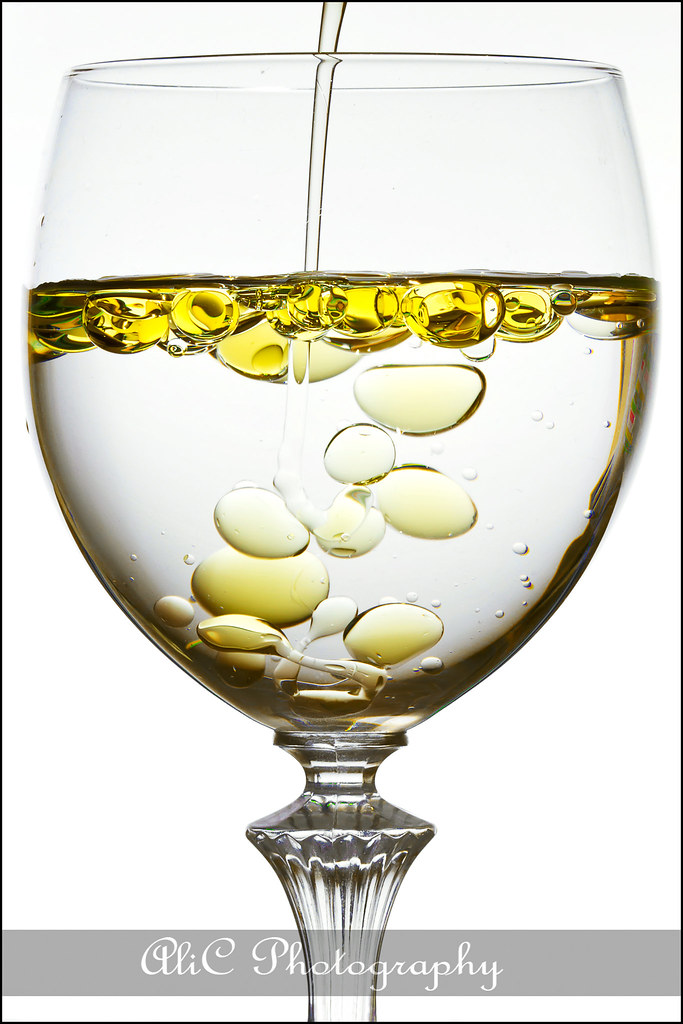
We can define density as the relationship between the weight and the volume of a substance or element. we can say that water is more dense than oil because with the same volume of each one, water is heavier. So, when we put both togethe in a glass, oil remains at the top and water goes down to the bottom.
density of water doesn't remain the same. It can change depending on temperature or salinity
- the rainbow:
The water drop is acting like a prism, except the light is being refracted at three different points (some of the light bounces off the back of the raindrop and back out to you as you watch). Each time the light beam bounces, it gets wider, and the rainbow you see is a combination of millions of these light beams coming back to you.

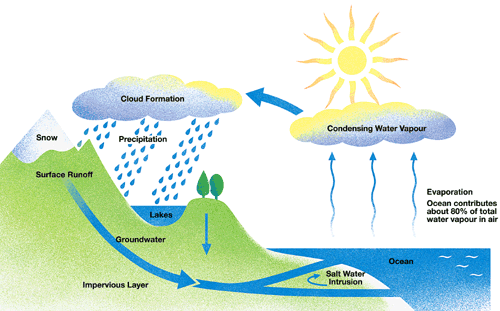
That picture explains what we know as the water cycle.
Almost 70% of the planet surface is water, the most importat element for life on earth.
The water cycle is the way the water moves all around the world, and do that in 6 basic processes:
Condensation - the opposite of evaporation. Condensation occurs when a gas is changed into a liquid.
Infiltration - Infiltration is an important process where rain water soaks into the ground, through the soil and underlying rock layers.
Runoff - Much of the water that returns to Earth as precipitation runs off the surface of the land, and flows down hill into streams, rivers, ponds and lakes.
Evaporation - the process where a liquid, in this case water, changes from its liquid state to a gaseous state.
Precipitation - When the temperature and atmospheric pressure are right, the small droplets of water in clouds form larger droplets and precipitation occurs. The raindrops fall to Earth.
Transpiration - As plants absorb water from the soil, the water moves from the roots through the stems to the leaves. Once the water reaches the leaves, some of it evaporates from the leaves, adding to the amount of water vapor in the air. This process of evaporation through plant leaves is called transpiration.
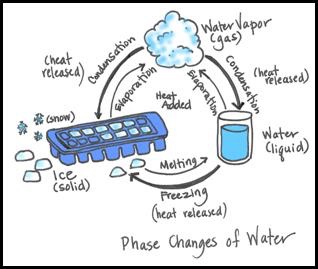
Another picture more complete.
The state changes of water.
There are three states where we can find water on earth: liquid (the water we drink), gaseous (a cloud) and solid (ice)
The water can change its state depending on the temperature and there is a name for each change:
- Water going from a solid to a liquid: Melting
- Water going from a liquid to a gas: Evaporation
- Water going from a solid to a gas: Sublimation
- Water going from a liquid to a solid: Freezing
- Water going from a gas to a liquid: Condensation
- Water going from a gas to a solid: Deposition
Short url: https://clilstore.eu/cs/5197